How to Effectively Draw Lewis Structures: Practical Tips for 2025
Drawing Lewis structures is a fundamental skill in chemistry that enables students and professionals alike to understand chemical bonding and molecular geometry. In this article, we’ll explore the intricacies of **drawing Lewis structures**, covering essential techniques, common mistakes, and practical exercises to enhance your understanding and application of Lewis theory.
The Basics of Lewis Structures
Before diving into the steps and techniques for **drawing Lewis structures**, it’s crucial to grasp the fundamentals. A Lewis structure is a visual representation of a molecule’s **electron dot structures**, showcasing how **valence electrons** are distributed among the atoms. It emphasizes **bond pairs**—the electrons involved in chemical bonds—and **lone pairs**, which are unshared electrons occupying atom valence shells. Mastering the basics includes understanding how **covalent bonds** form between atoms to fulfill the **octet rule**, which states that atoms tend to bond in such a way that they acquire eight electrons in their outermost shell, leading to greater chemical stability.
Understanding Valence Electrons
The first step in **drawing Lewis structures** involves determining the total number of **valence electrons** for a molecule or polyatomic ion. For instance, each element’s position on the periodic table helps identify its valence electrons—elements in the same column (group) have the same number. To calculate the total, simply add the valence electrons for all the atoms involved, including adjustments for any charges if it’s an ion. This comprehensive understanding of valence electrons enables accurate modeling of the molecular framework.
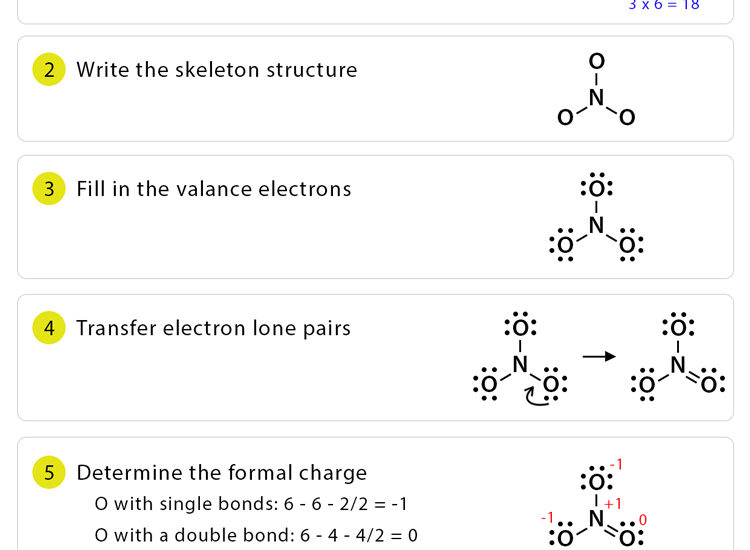
Steps for Drawing Lewis Structures
Here’s a step-by-step guide for **drawing Lewis structures** effectively:
- Count the total number of **valence electrons** from all atoms in the molecule or ion.
- Determine the central atom (typically the least electronegative) around which to arrange the other atoms.
- Connect outer atoms to the central atom with single bonds, using two electrons for each bond.
- Distribute any remaining electrons to satisfy the **octet rule** for outer atoms before adding to the central atom.
- If any atom still lacks a complete octet, form additional bonds by converting lone pairs into bond pairs.
Practical examples are often the most effective way to solidify these steps, and using visual aids, such as charts or software for chemistry, can greatly enhance understanding.
Common Mistakes in Drawing Lewis Structures
Like any skill, drawing Lewis structures comes with pitfalls. Understanding common mistakes can facilitate better learning and application. A prevalent error is misunderstanding the **octet rule**, particularly among elements that can accommodate more or fewer than eight electrons. Additionally, it’s key to avoid overemphasizing single bonds; sometimes, double or triple bonds are necessary for correct bonding representation. Such oversights can lead to inaccuracies in predicting molecular shapes and properties, crucial for both qualitative and quantitative analyses in chemical interactions.
Incorrect Bonding and Formal Charges
One common mistake involves committing to **bonding** configurations that do not minimize **formal charge** on atoms. The formal charge can be calculated using the formula:
\[ \text{Formal Charge} = \text{Valence Electrons} – \text{Nonbonding Electrons} – \frac{1}{2}(\text{Bonding Electrons}) \]
Situations where multiple arrangements yield valid structures require the consideration of resonance structures, ensuring the most stable configuration is chosen, which minimally disperses **formal charges** across the molecule.
Utilizing Visual Aids
To avoid mistakes, utilizing visual aids is highly effective. Online resources, instructional videos, and educational books that illustrate the principles of **Lewis structures** can be invaluable. These resources often offer interactive learning opportunities where students can practice and evaluate their understanding in real-time. Some software for chemistry even simulates **molecular interactions** and visualizes **bond angles** in three dimensions, providing a deeper comprehension of **molecular geometry**.
Advanced Techniques in Drawing Lewis Structures
Once you grasp the basics, you may want to delve into more complex aspects of **drawing Lewis structures**. Exploring the concept of **hybridization**, for example, reveals how various atomic orbitals combine to form **molecular orbitals** that dictate molecular shape and bonding characteristics. Additionally, understanding resonance can enhance the understanding of molecules where multiple Lewis structures coexist, contributing to its true electron distribution.
Hybridization and Molecular Shapes
Hybridization refers to the mixing of atomic orbitals to produce new hybrid orbitals, which determines the **bond angles** and **molecular shapes**. More advanced molecules may exhibit hybridization orders such as \(sp\), \(sp^2\), or \(sp^3\), influencing symmetry and geometry. Recognizing these configurations allows for precise predictions of how molecules will interact, hence aiding in the study of **chemical reactivity**.
Resonance Structures in Complex Molecules
Another advanced technique involves recognizing resonance structures—different Lewis representations of the same molecule that illustrate how electrons are shared. For example, benzene can be depicted through resonance, displaying its **aromatic compounds** characteristics. Understanding this concept not only aids in drawing but enhances comprehension of a molecule’s stability and its **intermolecular forces**.
Practical Applications of Lewis Structures
Understanding how to draw Lewis structures is not merely an academic exercise; it holds real-world significance in several fields. From predicting the **polarity** of molecules to assessing utility in organic or inorganic synthesis, Lewis structures serve as foundational tools in chemistry. As you become adept, consider applying these skills from the classroom to practical scenarios such as chemical modeling, materials science, and beyond.
Drawing Lewis Structures for Organic Compounds
When **drawing Lewis structures** for organic compounds, understanding functional groups is essential. Different functional groups possess different bonding characteristics, influencing how one constructs Lewis diagrams. An example could be **carboxylic acids**, which demonstrate both **covalent bonds** and can participate in hydrogen bonding due to their polarity. Recognizing the characteristics of functional groups facilitates clearer understanding and prediction of chemical behavior.
Predicting Molecular Behavior with Lewis Structures
Furthermore, Lewis structures provide invaluable insights into predicting both **molecular stability** and **chemical properties**. For instance, knowing the position of **lone pairs** vs **bond pairs** aids in mapping **dipole moments** and assessing intermolecular attractions, which are vital in determining boiling and melting points. Whether assessing **chemical interactions** in a lab or modeling theoretical compounds, an understanding of these structures leads to more informed, effective chemical conclusions.
Key Takeaways
- **Lewis structures** visually represent electron distribution and bonding in molecules.
- Accurate counting of **valence electrons** is essential in establishing a correct structure.
- Forming resonance structures and understanding hybridization enhances molecular understanding.
- Utilizing visual aids and software increases learning efficiency and accuracy.
- Practical applications of Lewis structures extend into various fields, particularly healthcare and material science.
FAQ
1. What are the common mistakes made while drawing Lewis structures?
Common mistakes include miscalculating the total number of **valence electrons**, neglecting the concept of **formal charge**, and failing to recognize the need for resonance structures. Specific elements that exceed the **octet rule** often result in inaccuracies, emphasizing the need for careful consideration of various bonding configurations.
2. How do I determine if a molecule is polar or non-polar using Lewis structures?
By analyzing the arrangement of **bond pairs** and **lone pairs** around the central atom from the Lewis structure, one can assess molecular symmetry. If the molecule possesses an overall symmetry alongside evenly distributed electronegativity, it is deemed **non-polar**; otherwise, it is **polar** due to unequal electron sharing that generates a dipole moment.
3. What role do formal charges play in Lewis structures?
Formal charges aid in determining the most stable Lewis structure by indicating electron distribution and bonding quality. Structures with minimized formal charges on atoms generally depict the lowest energy configurations, thereby guiding further understanding of a molecule’s **chemical properties** and reactivity.
4. How can resonance structures help in understanding molecular behavior?
Resonance structures illustrate the delocalization of electrons across multiple configurations, indicating that the actual structure is a hybrid of these representations. Recognizing this can provide insights into the **stability** and reactivity of complex molecules, guiding predictions of how they might behave in chemical reactions.
5. What software resources can enhance learning about Lewis structures?
Several interactive software programs and online platforms, such as ChemDraw or Molecular Workbench, offer tools for **visualizing molecular shapes** and simulating various chemical interactions. Such resources enhance comprehension and provide practical applications of Lewis structures in numerous fields.
6. How can drawing methods impact my understanding of molecular geometry?
Utilizing different **drawing techniques**, such as **3D modeling**, directly correlates with enhanced understanding of **molecular geometry**. Recognizing how **bond angles** and electron pair arrangements affect molecular structures underlies critical learning pathways in grasping wider concepts in chemical bonding theories.
7. Why are **Lewis structures** important in organic chemistry?
In organic chemistry, **Lewis structures** are essential for visualizing **functional groups** present in molecules. They serve as foundational tools for predicting behaviors, understanding properties, and assisting in mechanistic interpretations during synthesis, leading to more informed predictions about chemical interactions and structures.
Make sure to utilize the image links provided to enrich understanding with visual representations of the concepts discussed throughout the article.