How to Calculate Specific Heat for Improved Results in 2025
Understanding how to calculate **specific heat** is crucial for various scientific applications, from laboratory experiments to industrial processes. Specific heat is defined as the amount of heat required to change the temperature of a unit mass of a substance by one degree Celsius (°C). This article provides a comprehensive guide on the **specific heat formula**, its application in different materials, and practical tips to maximize your calculations.
Understanding Specific Heat and Its Importance
Before diving into calculations, it’s important to grasp what specific heat capacity entails. Specific heat capacity, often referred to simply as specific heat, measures a substance’s ability to store thermal energy. This capability varies significantly across different materials, impacting numerous fields, including chemistry, engineering, and environmental science. For example, the **specific heat of water** is notably high, which plays a critical role in climate regulation and metabolic processes. Understanding the influence of specific heat can help optimize energy usage in various applications, such as designing heat exchangers and improving thermal insulation.
Defining Specific Heat in Physics and Chemistry
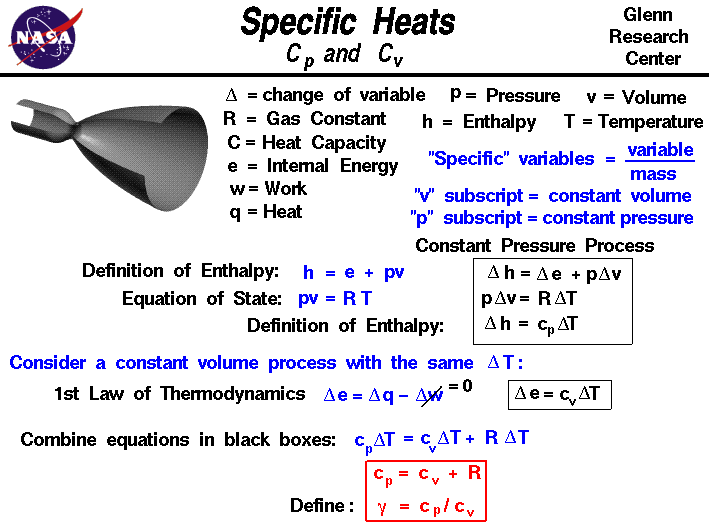
The scientific **definition of specific heat** is the amount of heat needed per unit mass to raise its temperature by a specified amount. It is commonly expressed in units of joules per gram per degree Celsius (J/g°C). In practical terms, when conducting a **specific heat calculation**, you will often encounter the formula:
Q = mcΔT
In this equation, Q represents the heat added or removed (in joules), m is the mass (in grams), c is the specific heat capacity, and ΔT is the change in temperature. Understanding this relationship is foundational in **calorimetry** experiments—where heat transfer is studied to assess thermal properties of various substances.
Different Types of Specific Heat
Specific heat can differ significantly depending on the substance, categorized broadly into specific heat of solids, liquids, and gases. The **specific heat of metals**, for instance, tends to be lower than that of liquids and gases, indicating that metals heat up and cool down more quickly compared to water or air. Sources such as **specific heat data tables** can provide you with the values necessary for accurate computations, enabling professionals and students alike to compare how different materials respond to thermal energy.
Calculating Specific Heat: An In-Depth Guide
Calculating specific heat is a straightforward process if you have accurate measurements. Using the previously mentioned formula, you can derive specific heat for various substances by measuring heat transfer during a thermal event. Whether you are engaged in **specific heat laboratory experiments** or simply exploring **specific heat in real-world applications**, following structured steps improves accuracy.
Steps for Specific Heat Calculation
1. **Gather necessary data**: You will need the mass of the substance, the initial and final temperatures, and the heat added or removed during the temperature change.
2. **Measure the temperature change**: Subtract the initial temperature from the final temperature to find ΔT. This value can be critical, especially when calculating the **specific heat of gases** which often require precision.
3. **Apply the formula**: Plug the values into the specific heat formula (Q = mcΔT) and solve for ‘c’ to find the specific heat capacity.
4. **Document results**: Record your findings and compare them with existing values (like the **specific heat of water** or the **specific heat of aluminum**) to validate your results.
Managing Heat Transfer and Assessing Precision
During specific heat calculations, managing potential errors in measurements is vital. **Heat transfer** can be influenced by numerous factors, such as environmental conditions, phase changes in materials, and instrument calibration. Utilizing high-quality thermometers and calorimeters can significantly enhance the reliability of your readings. Additionally, conducting multiple trials and averaging the results can mitigate random errors, establishing a more dependable understanding of specific heat for various materials.
Practical Applications of Specific Heat
Many industries utilize **specific heat** concepts daily, from chemical engineering to environmental management. The ability to accurately calculate specific heat can lead to more efficient processes, such as in energy conservation strategies and material selection for thermal applications. Examining the **specific heat values** of different substances can also aid in material design focused on thermal conductivity and energy storage.
Real-World Applications
For example, **specific heat in engineering** is critical when designing HVAC systems, where understanding air’s specific heat enables more efficient heating and cooling strategies. Additionally, studying the **specific heat of organic compounds** is essential for industries in food production, pharmaceuticals, and materials science that depend on precise thermal management. By analyzing specific heat, engineers and scientists can create better insulated materials that save energy and enhance performance.
Case Studies and Empirical Studies on Specific Heat
Numerous studies illustrate the significance of specific heat in natural processes, notably in climate science. The **specific heat of water** stabilizes temperatures in oceans and weather patterns, influencing ecosystems around the world. Research involving **specific heat variations across states (solid, liquid, gas)** provides insight into how climate change impacts thermal dynamics in different environments.
Key Takeaways
- **Specific heat** is fundamental in understanding thermal energy transfer and temperature changes across various materials.
- The main formula Q = mcΔT is crucial for calculating specific heat accurately.
- Specific heat variations across different substances dictate their practical applications, influencing industries such as engineering and environmental management.
- Precision in measurement tools can dramatically affect the reliability of specific heat calculations.
- Understanding specific heat is not only essential in academia but also in real-world applications affecting technology and resource management.
FAQ
1. What is the specific heat of water compared to other substances?
The **specific heat of water** is about 4.18 J/g°C, which is relatively high compared to metals like copper (0.39 J/g°C). This property allows water to regulate temperature effectively in various environmental and biological systems.
2. How can I find specific heat values for different materials?
You can find **specific heat values** in various resources, including scientific literature, dedicated data tables, or online databases focused on material properties. These tables often include values for solids, liquids, and gases.
3. What role does specific heat play in calorimetry experiments?
In **calorimetry**, specific heat is fundamental for measuring thermal energy changes during reactions or phase transitions. Understanding specific heat allows for the precise calculations of energy transfer between substances.
4. How does specific heat affect temperature change?
**Specific heat** directly influences how much a temperature will change for a material when a certain amount of heat is added. Materials with high specific heat require more energy to change temperature than materials with low specific heat.
5. Can I calculate specific heat using common kitchen items?
Yes! You can perform simple experiments using kitchen items. For example, measuring the temperature change of water when heating a metal object can yield insights into the **specific heat** of the material involved.
6. What is the significance of **specific heat** in thermodynamics?
In thermodynamics, **specific heat** plays a critical role in energy conservation calculations and understanding heat transfer mechanisms, providing insights for systems ranging from engines to refrigerators.
7. How does specific heat relate to thermal expansion?
**Specific heat** and thermal expansion are interconnected; materials with high specific heat tend to experience minimal changes in temperature and, consequently, less thermal expansion compared to materials with lower specific heat.